Solketal Production by Glycerol Acetalization Using Amberlyst-15 Catalyst
Abstract
Glycerol, as a by-product of biodiesel production, has recently increased due to the rapid growth of the biodiesel industry. Glycerol utilization is needed to increase the added value of glycerol. Glycerol can be converted to solketal, which can be used as a green fuel additive to enhance an octane or cetane number. Conversion of glycerol to solketal was conducted via acetalization reaction with acetone using amberlyst-15 as the catalyst. The objective of present study was to investigate the effect of some operation conditions on glycerol conversion. Furthermore, it also aimed to develop a kinetic model of solketal synthesis with amberlyst-15 resins. The experiment was conducted in a batch reactor, equipped with cooling water, thermometer, stirrer, and a water bath. The variables that have been investigated in the present work were reaction temperature, reactants molar ratio, catalyst loading, and stirrer speed for 3 hours of reaction time. Temperatures, reactants molar ratio, and stirrer speed appeared to have a significant impact on glycerol conversion, where the higher values led to higher conversion. On the other hand, in the presence of catalyst, the increase of catalyst loading has a less significant impact on glycerol conversion. The results showed that the highest glycerol conversion was 68.75%, which was obtained at 333 K, the reactant’s molar ratio was 4, the amount of catalyst was 1 wt%, and stirrer speed of 500 rpm. Based on the pseudo-homogeneous kinetic model, the resulting kinetic model suitable for this glycerol capitalization.
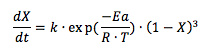
The value of parameters k and Ea were 1.6135 108 min-1 and 62.226 kJ mol-1,respectively. The simulation model generally fits the experimental data reasonably well in the temperature range of 313-333 K.
References
2. Aghbashlo, M., Tabatabaei, M., Hosseinpour, S., Rastegari, H., and Ghaziaskar, H. S., (2018). “Multi-objective exergy-based optimization of continuous glycerol ketalization to synthesize solketal as a biodiesel additive in subcritical acetone,” Energy Convers. Manag., 160, 251–261.
3. Barbosa, S. L., Lima, P. C., dos Santos, W. T. P., Klein, S. I., Clososki, G. C. and Caires, F. J. (2019). “Oxygenated biofuels: Synthesis of fatty acid solketal esters with a mixture of sulfonated silica and (Bu4N)(BF4) catalyst,” Catal. Commun.,120, 76–79,.
4. Castanheiro, J. E., Vital, J., Fonseca, I. M., Ramos, A. M., (2015). “Glycerol conversion into biofuel additives by acetalization with pentanal over heteropolyacids immobilized on zeolites” , Cat. Today, 2019. https://doiorg/10.1016/j.cattod.2019.04.048
5. da Silva, M. J., de Ávila Rodrigues, F. and Júlio, A. A. (2017). “SnF2-catalyzed glycerol ketalization: A friendly environmentally process to synthesize solketal at room temperature over on solid and reusable Lewis acid,” Chem. Eng. J., 307, 828–835.
6. Gadamseti, S., Rajan, N. P., Rao, G. S., Chary, K. V. R., (2015). “Acetalization of glycerol with acetone to bio fuel additive over supported molyb- denum phosphate catalysts, “ J. Mol. Cat. A: Chemical, 410, 49 – 57.
7. Huda, E. N., (2019). “Pemanfaatan Gliserol Hasil Samping Produksi Biodiesel untuk Pembuatan Solketal: Pengaruh Suhu dan Konsentrasi Katalis,” Research Report of Chemical Reaction Engineering and Catalysis Lab., Dep. Chem. Eng. Univ. Gadjah Mada, Yogyakarta, Indonesia,.
8. Khayoon, M. S., and Hameed, B. H., (2013). “Solventless acetalization of glycerol with acetone to fuel oxygenates over Ni–Zr supported on mesoporous activated carbon catalyst,” Appl. Catal. A Gen., 464–465, 191–199.
9. Li, X., Jiang, Y., Zhou, R., and Hou, Z., (2019). “Layered α-zirconium phosphate: An efficient catalyst for the synthesis of solketal from glycerol,” Appl. Clay Sci., 174, 120–126.
10. Manjunathan, P., Maradur, S. P., Halgeri, A. B., and Shanbhag, G. V., (2015). “Room temperature synthesis of solketal from acetalization of glycerol with acetone: Effect of crystallite size and the role of acidity of beta zeolite,” J. Mol. Catal. A Chem., 396,. 47–54.
11. Nanda, M. R., Yuan, Z., Qin, W., Ghaziaskar, H. S., Poirier, M. A., and Xu, C.C., (2014). “Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive,” Fuel, 117, 470–477.
12. Priya, S. S., Selvakannan, P. R., Chary, K. V. R., Kantam, M. L., and Bhargava, S. K., (2017). “Solvent-free microwave-assisted synthesis of solketal from glycerol using transition metal ions promoted mordenite solid catalysts,” Molecular Catalysis, 434, 184-193.
13. Qadariyah, L., Bhuana, D.S., Selaksa, R., As Shodiq, J., and Mahfud, M.. "Biodiesel production from Calophyllum inophyllum using base lewis catalyst”, ASEAN Journal of Chemical Engingeering, 18, 53-59.
14. Reddy, P. S., Sudarsanam P., Mallesham, G., Raju, G., and Reddy, B. M., (2011). “Acetalization of glycerol with acetone over zirconia and promoted zirconia catalysts under mild reaction conditions,” J. Ind. Eng. Chem., 17, 377–381.
15. Rossa, V., Pessanha, Y. S. P., Diaz, G. C., Camara, L. D. T., Pergher, S. B., C. Aranda, D. A. G., (2017). “Reaction kinetic study of solketal production from glycerol ketalization with acetone,” Ind. Eng. Che. Res., vol 56, pp 479 – 488.
16. Souza, T. E., Padula, I. D., Teodoro, M. M. G., Chagas, P., Resende, J. M., Souza, P. P., and Oliveira, L. C. A., (2015). “Amphiphilic property of niobium oxyhydroxide for waste glycerol conversion to produce solketal,” Catal. Today, 254, 83–89.
17. Sulistyo, H., Hapsari, I., Budhijanto, Sediawan, W. B., Rahayu, S. S., and Azis, M. M., (2019). “Heterogeneous catalytic reaction of glycerol with acetone for solketal production,” MATEC Web Conf., 268, 7004.
18. Sulistyo, H., Priadana, D. P., Fitriandini, Y. W., Ariyanto, T., Azis, M. M., (2020). “Utilization of glycerol by ketalization reaction with acetone to produce solketal using indion 225Na as catalyst,” Int. J. Tech, 11, 190 – 199.
19. Utami, T. S., (2019). “Pemanfaatan Glicerol Hasil Samping Produksi Biodiesel untuk Pembuatan Solketal: Pengaruh Rasio Reaktan dan Kecepatan Pengadukan,” Research Report of Chemical Reaction Engineering and Catalysis Lab., Dep. Chem. Eng. Univ. Gadjah Mada, Yogyakarta, Indonesia,
20. Vicente, G., Melero, J. A., Morales, G., Paniagua, M., and Martín, E., (2010). “Acetalization of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas,” Green Chem., 12, 899–907.
Copyright holder for articles is ASEAN Journal of Chemical Engineering. Articles published in ASEAN J. Chem. Eng. are distributed under a Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) license.
Authors agree to transfer all copyright rights in and to the above work to the ASEAN Journal of Chemical Engineering Editorial Board so that the Editorial Board shall have the right to publish the work for non-profit use in any media or form. In return, authors retain: (1) all proprietary rights other than copyright; (2) re-use of all or part of the above paper in their other work; (3) right to reproduce or authorize others to reproduce the above paper for authors’ personal use or for company use if the source and the journal copyright notice is indicated, and if the reproduction is not made for the purpose of sale.



